Efficacy of High- and Low-Dose, Highly Bioavailable Curcumin (CurcuRouge®) for Treating Knee Osteoarthritis: A Randomized, Double-Blind, Placebo-Controlled Prospective Study
DOI:
https://doi.org/10.52845/CMI/2023-4-3-3Keywords:
Knee osteoarthritis, treatment, safety, highly bioavailable curcumin, CurcuRouge○RAbstract
【Purposes】
To evaluate the clinical effects of orally administered CurcuRougeR in patients with knee osteoarthritis (OA) after 12 weeks of treatment.
【Methods】
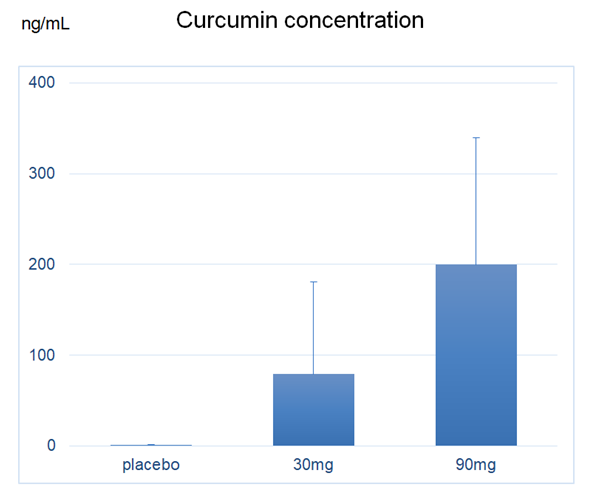
In this randomized, double-blind, placebo-controlled, prospective clinical study. 90 patients over the age 40 with knee OA of Kellgren-Lawrence (KL) Grade II or III were enrolled. A placebo or CurcuRougeR containing 60 mg or 180 mg/day of curcumin was administered orally every day for 12 weeks. To monitor adverse events, blood biochemical analyses were performed before and after 12 weeks of each intervention. The patients’ knee symptoms were evaluated for 12 weeks using the Japanese Knee Osteoarthritis Measure (JKOM) criteria, a knee pain visual analog scale (VAS), the Japanese Orthopedic Association (JOA) knee scoring system, the need for nonsteroidal anti-inflammatory drugs (NSAIDs) and a timed up-and-go test (TUG).
【Results】
The declining trend in NSAIDs needs was significantly greater in high-dose CurcuRougeR group than in placebo group. The decrease of highly sensitive CRP was significantly greater in high-dose of CurcuRouge® than placebo group. In patients with KL Grade II, JOA and VAS scores improved significantly more in the high-dose CurcuRougeR group than in the placebo group. We didn’t find CurcuRouge® related adverse event during the study period.
【Conclusion】
CurcuRougeR has succeeded in proofing a potential for treating human knee OA, particularly at an early stage.
Article Metrics Graph